Primary Cell
The dry cell is the most common type of primary-cell battery and is similar in its characteristics to that of an electrolytic cell. This type of a battery is basically designed with a metal electrode or graphite rod acting as the cathode (+) terminal, immersed in an electrolytic paste. This electrode/electrolytic build-up is then encased in a metal container, usually made of zinc, which itself acts as the anode (−) terminal. When the battery is in a discharge condition an electrochemical reaction takes place resulting in one of the metals being consumed. Because of this consumption, the charging process is not reversible. Attempting to reverse the chemical reaction in a primary cell by way of recharging is usually dangerous and can lead to a battery explosion.These batteries are commonly used to power items such as flashlights. The most common primary cells today are found in alkaline batteries, silver-oxide, and lithium batteries. The earlier carbon-zinc cells, with a carbon post as cathode and a zinc shell as anode were once prevalent but are not as common.
Secondary Cell
A secondary cell is any kind of electrolytic cell in which the electrochemical reaction that releases energy is reversible. The lead-acid car battery is a secondary-cell battery. The electrolyte is sulfuric acid (battery acid), the positive electrode is lead peroxide, and the negative electrode is lead. A typical lead-acid battery consists of six lead-acid cells in a case. Each cell produces 2 volts, so the whole battery produces a total of 12 volts.Other commonly used secondary cell chemistry types are nickel cadmium (NiCd), nickel metal hydride (NiMH), lithium ion (Li-ion), and lithium ion polymer (Li-ion polymer).
Lead-acid batteries used in aircraft are similar to automobile batteries. The lead acid battery is made up of a series of identical cells each containing sets of positive and negative plates. Figure 1 illustrates each cell contains positive plates of lead dioxide (PbO2), negative plates of spongy lead, and electrolyte (sulfuric acid and water). A practical cell is constructed with many more plates than just two in order to get the required current output. All positive plates are connected together as well as all the negatives. Because each positive plate is always positioned between two negative plates, there are always one or more negative plates than positive plates.
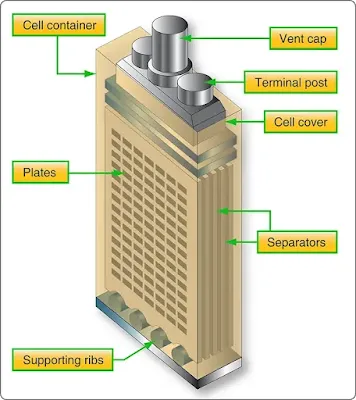 |
| Figure 1. Lead-acid cell construction |
Between the plates are porous separators that keep the positive and negative plates from touching each other and shorting out the cell. The separators have vertical ribs on the side facing the positive plate. This construction permits the electrolyte to circulate freely around the plates. In addition, it provides a path for sediment to settle to the bottom of the cell.
Each cell is seated in a hard rubber casing through the top of which are terminal posts and a hole into which a nonspill vent cap is screwed. The hole provides access for testing the strength of the electrolyte and adding water. The vent plug permits gases to escape from the cell with a minimum of leakage of electrolyte, regardless of the position the airplane might assume. [Figure 2] In level flight, the lead weight permits venting of gases through a small hole. In inverted flight, this hole is covered by the lead weight.
The individual cells of the battery are connected in series by means of cell straps. [Figure 3] The complete assembly is enclosed in an acid resisting metal container (battery box), which serves as electrical shielding and mechanical protection. The battery box has a removable top. It also has a vent tube nipple at each end. When the battery is installed in an airplane, a vent tube is attached to each nipple. One tube is the intake tube and is exposed to the slipstream. The other is the exhaust vent tube and is attached to the battery drain sump, which is a glass jar containing a felt pad moistened with a concentrated solution of sodium bicarbonate (baking soda). With this arrangement, the airstream is directed through the battery case where battery gases are picked up, neutralized in the sump, and then expelled overboard without damage to the airplane.
To facilitate installation and removal of the battery in some aircraft, a quick disconnect assembly is used to connect the power leads to the battery. This assembly attaches the battery leads in the aircraft to a receptacle mounted on the side of the battery. [Figure 4] The receptacle covers the battery terminal posts and prevents accidental shorting during the installation and removal of the battery. The plug consists of a socket and a handwheel with a course pitch thread. It can be readily connected to the receptacle by the handwheel. Another advantage of this assembly is that the plug can be installed in only one position, eliminating the possibility of reversing the battery leads.
The voltage of lead acid cell is approximately two volts in order to attain the voltage required for the application. Each cell is then connected in series with heavy gauge metal straps to form a battery. In a typical battery, such as that used in an aircraft for starting, the voltage required is 12 or 24 volts. This voltage is achieved by connecting six cells or twelve cells respectively together in series and enclosing them in one plastic box.
Each cell containing the plates are filled with an electrolyte composed of sulfuric acid and distilled water with a specific gravity of 1.270 at 60 °F. This solution contains positive hydrogen ions and negative sulfate (SO4) ions that are free to combine with other ions and form a new chemical compound. When the cell is discharged, electrons leave the negative plate and flow to the positive plates where they cause the lead dioxide (PbO2) to break down into negative oxygen ions and positive lead ions. The negative oxygen ions join with positive hydrogen ions from the sulfuric acid and form water (H2O). The negative sulfate ions join with the lead ions in both plates and form lead sulfate (PbSO4). After the discharge, the specific gravity changes to about 1.150.
[ad-mid]
The most common battery rating is the amp-hour rating. This is a unit of measurement for battery capacity. It is determined by multiplying a current flow in amperes by the time in hours that the battery is being discharged.
A battery with a capacity of 1 amp-hour should be able to continuously supply a current of 1 amp to a load for exactly 1 hour, or 2 amps for 1⁄2 hour, or 1⁄3 amp for 3 hours, etc., before becoming completely discharged. Actually, the ampere-hour output of a particular battery depends on the rate at which it is discharged. Heavy discharge current heats the battery and decreases its efficiency and total ampere-hour output. For airplane batteries, a period of 5 hours has been established as the discharge time in rating battery capacity. However, this time of 5 hours is only a basis for rating and does not necessarily mean the length of time during which the battery is expected to furnish current. Under actual service conditions, the battery can be completely discharged within a few minutes, or it may never be discharged if the generator provides sufficient charge.
The ampere-hour capacity of a battery depends upon its total effective plate area. Connecting batteries in parallel increases ampere-hour capacity. Connecting batteries in series increases the total voltage but not the ampere-hour capacity.
The most commonly used hydrometer consists of a small sealed glass tube weighted at its lower end so it floats upright. [Figure 5] Within the narrow stem of the tube is a paper scale with a range of 1.100 to 1.300. When a hydrometer is used, a quantity of electrolyte sufficient to float the hydrometer is drawn up into the syringe. The depth to which the hydrometer sinks into the electrolyte is determined by the density of the electrolyte, and the scale value indicated at the level of the electrolyte is its specific gravity. The more dense the electrolyte, the higher the hydrometer floats; therefore, the highest number on the scale (1.300) is at the lower end of the hydrometer scale.
In a new, fully-charged aircraft storage battery, the electrolyte is approximately 30 percent acid and 70 percent water (by volume) and is 1.300 times as heavy as pure water. During discharge, the solution (electrolyte) becomes less dense and its specific gravity drops below 1.300. A specific gravity reading between 1.300 and 1.275 indicates a high state of charge; between 1.275 and 1.240, a medium state of charge; and between 1.240 and 1.200, a low state of charge. Aircraft batteries are generally of small capacity but are subject to heavy loads. The values specified for state of charge are therefore rather high. Hydrometer tests are made periodically on all storage batteries installed in aircraft. An aircraft battery in a low state of charge may have perhaps 50 percent charge remaining, but is nevertheless considered low in the face of heavy demands that would soon exhaust it. A battery in such a state of charge is considered in need of immediate recharging.
When a battery is tested using a hydrometer, the temperature of the electrolyte must be taken into consideration. The specific gravity readings on the hydrometer vary from the actual specific gravity as the temperature changes. No correction is necessary when the temperature is between 70 °F and 90 °F, since the variation is not great enough to consider. When temperatures are greater than 90 °F or less than 70 °F, it is necessary to apply a correction factor. Some hydrometers are equipped with a correction scale inside the tube. With other hydrometers, it is necessary to refer to a chart provided by the manufacturer. In both cases, the corrections should be added to, or subtracted from the reading shown on the hydrometer.
The specific gravity of a cell is reliable only if nothing has been added to the electrolyte except occasional small amounts of distilled water to replace that lost as a result of normal evaporation. Always take hydrometer readings before adding distilled water, never after. This is necessary to allow time for the water to mix thoroughly with the electrolyte and to avoid drawing up into the hydrometer syringe a sample that does not represent the true strength of the solution.
Exercise extreme care when making the hydrometer test of a lead-acid cell. Handle the electrolyte carefully because sulfuric acid burns clothing and skin. If the acid does contact the skin, wash the area thoroughly with water and then apply bicarbonate of soda.
[ad-long]
Batteries are charged by either the constant voltage or constant current method. In the constant voltage method [Figure 6A], a motor generator set with a constant, regulated voltage forces the current through the battery. In this method, the current at the start of the process is high but automatically tapers off, reaching a value of approximately 1 ampere when the battery is fully charged. The constant voltage method requires less time and supervision than does the constant current method.
In the constant current method [Figure 6B], the current remains almost constant during the entire charging process. This method requires a longer time to charge a battery fully and, toward the end of the process, presents the danger of overcharging, if care is not exercised.
In the aircraft, the storage battery is charged by direct current from the aircraft generator system. This method of charging is the constant voltage method, since the generator voltage is held constant by use of a voltage regulator.
When a storage battery is being charged, it generates a certain amount of hydrogen and oxygen. Since this is an explosive mixture, it is important to take steps to prevent ignition of the gas mixture. Loosen the vent caps and leave in place. Do not permit open flames, sparks, or other sources of ignition in the vicinity. Before disconnecting or connecting a battery to the charge, always turn off the power by means of a remote switch.
Each cell is seated in a hard rubber casing through the top of which are terminal posts and a hole into which a nonspill vent cap is screwed. The hole provides access for testing the strength of the electrolyte and adding water. The vent plug permits gases to escape from the cell with a minimum of leakage of electrolyte, regardless of the position the airplane might assume. [Figure 2] In level flight, the lead weight permits venting of gases through a small hole. In inverted flight, this hole is covered by the lead weight.
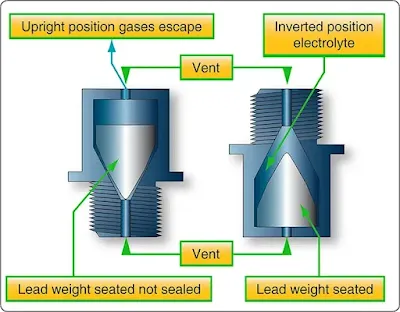 |
| Figure 2. Nonspill battery vent plug |
The individual cells of the battery are connected in series by means of cell straps. [Figure 3] The complete assembly is enclosed in an acid resisting metal container (battery box), which serves as electrical shielding and mechanical protection. The battery box has a removable top. It also has a vent tube nipple at each end. When the battery is installed in an airplane, a vent tube is attached to each nipple. One tube is the intake tube and is exposed to the slipstream. The other is the exhaust vent tube and is attached to the battery drain sump, which is a glass jar containing a felt pad moistened with a concentrated solution of sodium bicarbonate (baking soda). With this arrangement, the airstream is directed through the battery case where battery gases are picked up, neutralized in the sump, and then expelled overboard without damage to the airplane.
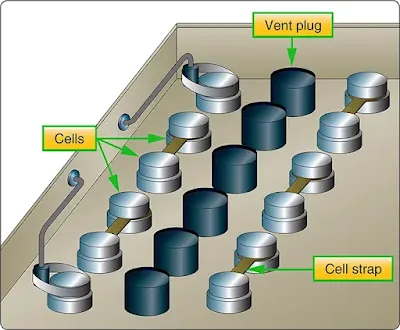 |
| Figure 3. Connection of storage battery |
To facilitate installation and removal of the battery in some aircraft, a quick disconnect assembly is used to connect the power leads to the battery. This assembly attaches the battery leads in the aircraft to a receptacle mounted on the side of the battery. [Figure 4] The receptacle covers the battery terminal posts and prevents accidental shorting during the installation and removal of the battery. The plug consists of a socket and a handwheel with a course pitch thread. It can be readily connected to the receptacle by the handwheel. Another advantage of this assembly is that the plug can be installed in only one position, eliminating the possibility of reversing the battery leads.
 |
| Figure 4. A battery quick-disconnect assembly |
The voltage of lead acid cell is approximately two volts in order to attain the voltage required for the application. Each cell is then connected in series with heavy gauge metal straps to form a battery. In a typical battery, such as that used in an aircraft for starting, the voltage required is 12 or 24 volts. This voltage is achieved by connecting six cells or twelve cells respectively together in series and enclosing them in one plastic box.
Each cell containing the plates are filled with an electrolyte composed of sulfuric acid and distilled water with a specific gravity of 1.270 at 60 °F. This solution contains positive hydrogen ions and negative sulfate (SO4) ions that are free to combine with other ions and form a new chemical compound. When the cell is discharged, electrons leave the negative plate and flow to the positive plates where they cause the lead dioxide (PbO2) to break down into negative oxygen ions and positive lead ions. The negative oxygen ions join with positive hydrogen ions from the sulfuric acid and form water (H2O). The negative sulfate ions join with the lead ions in both plates and form lead sulfate (PbSO4). After the discharge, the specific gravity changes to about 1.150.
[ad-mid]
Battery Ratings
The voltage of a battery is determined by the number of cells connected in series to form the battery. Although the voltage of one lead-acid cell just removed from a charger is approximately 2.2 volts, a lead-acid cell is normally rated at approximately 2 volts. A battery rated at 12 volts consists of 6 lead-acid cells connected in series, and a battery rated at 24 volts is composed of 12 cells.The most common battery rating is the amp-hour rating. This is a unit of measurement for battery capacity. It is determined by multiplying a current flow in amperes by the time in hours that the battery is being discharged.
A battery with a capacity of 1 amp-hour should be able to continuously supply a current of 1 amp to a load for exactly 1 hour, or 2 amps for 1⁄2 hour, or 1⁄3 amp for 3 hours, etc., before becoming completely discharged. Actually, the ampere-hour output of a particular battery depends on the rate at which it is discharged. Heavy discharge current heats the battery and decreases its efficiency and total ampere-hour output. For airplane batteries, a period of 5 hours has been established as the discharge time in rating battery capacity. However, this time of 5 hours is only a basis for rating and does not necessarily mean the length of time during which the battery is expected to furnish current. Under actual service conditions, the battery can be completely discharged within a few minutes, or it may never be discharged if the generator provides sufficient charge.
The ampere-hour capacity of a battery depends upon its total effective plate area. Connecting batteries in parallel increases ampere-hour capacity. Connecting batteries in series increases the total voltage but not the ampere-hour capacity.
Life Cycle of a Battery
Battery life cycle is defined as the number of complete charge/ discharge cycles a battery can perform before its normal charge capacity falls below 80 percent of its initial rated capacity. Battery life can vary anywhere from 500 to 1,300 cycles. Various factors can cause deterioration of a battery and shorten its service life. The first is over-discharging, which causes excess sulfation; second, too-rapid charging or discharging that results in overheating of the plates and shedding of active material. The accumulation of shed material, in turn, causes shorting of the plates and results in internal discharge. A battery that remains in a low or discharged condition for a long period of time may be permanently damaged. The deterioration can continue to a point where cell capacity can drop to 80 percent after 1,000 cycles. In many cases, the cell can continue working to nearly 2,000 cycles but with a diminished capacity of 60 percent of its original state.Lead-Acid Battery Testing Methods
The state of charge of a storage battery depends upon the condition of its active materials, primarily the plates. However, the state of charge of a battery is indicated by the density of the electrolyte and is checked by a hydrometer, an instrument that measures the specific gravity (weight as compared with water) of liquids.The most commonly used hydrometer consists of a small sealed glass tube weighted at its lower end so it floats upright. [Figure 5] Within the narrow stem of the tube is a paper scale with a range of 1.100 to 1.300. When a hydrometer is used, a quantity of electrolyte sufficient to float the hydrometer is drawn up into the syringe. The depth to which the hydrometer sinks into the electrolyte is determined by the density of the electrolyte, and the scale value indicated at the level of the electrolyte is its specific gravity. The more dense the electrolyte, the higher the hydrometer floats; therefore, the highest number on the scale (1.300) is at the lower end of the hydrometer scale.
 |
| Figure 5. Hydrometer (specific gravity readings) |
In a new, fully-charged aircraft storage battery, the electrolyte is approximately 30 percent acid and 70 percent water (by volume) and is 1.300 times as heavy as pure water. During discharge, the solution (electrolyte) becomes less dense and its specific gravity drops below 1.300. A specific gravity reading between 1.300 and 1.275 indicates a high state of charge; between 1.275 and 1.240, a medium state of charge; and between 1.240 and 1.200, a low state of charge. Aircraft batteries are generally of small capacity but are subject to heavy loads. The values specified for state of charge are therefore rather high. Hydrometer tests are made periodically on all storage batteries installed in aircraft. An aircraft battery in a low state of charge may have perhaps 50 percent charge remaining, but is nevertheless considered low in the face of heavy demands that would soon exhaust it. A battery in such a state of charge is considered in need of immediate recharging.
When a battery is tested using a hydrometer, the temperature of the electrolyte must be taken into consideration. The specific gravity readings on the hydrometer vary from the actual specific gravity as the temperature changes. No correction is necessary when the temperature is between 70 °F and 90 °F, since the variation is not great enough to consider. When temperatures are greater than 90 °F or less than 70 °F, it is necessary to apply a correction factor. Some hydrometers are equipped with a correction scale inside the tube. With other hydrometers, it is necessary to refer to a chart provided by the manufacturer. In both cases, the corrections should be added to, or subtracted from the reading shown on the hydrometer.
The specific gravity of a cell is reliable only if nothing has been added to the electrolyte except occasional small amounts of distilled water to replace that lost as a result of normal evaporation. Always take hydrometer readings before adding distilled water, never after. This is necessary to allow time for the water to mix thoroughly with the electrolyte and to avoid drawing up into the hydrometer syringe a sample that does not represent the true strength of the solution.
Exercise extreme care when making the hydrometer test of a lead-acid cell. Handle the electrolyte carefully because sulfuric acid burns clothing and skin. If the acid does contact the skin, wash the area thoroughly with water and then apply bicarbonate of soda.
[ad-long]
Lead-Acid Battery Charging Methods
Passing direct current through the battery in a direction opposite to that of the discharge current may charge a storage battery. Because of the internal resistance (IR) in the battery, the voltage of the external charging source must be greater than the open circuit voltage. For example, the open circuit voltage of a fully charged 12 cell, lead-acid battery is approximately 26.4 volts (12 × 2.2 volts), but approximately 28 volts are required to charge it. This larger voltage is needed for charging because of the voltage drop in the battery caused by the internal resistance. Hence, the charging voltage of a lead-acid battery must equal the open circuit voltage plus the IR drop within the battery (product of the charging current and the internal resistance).Batteries are charged by either the constant voltage or constant current method. In the constant voltage method [Figure 6A], a motor generator set with a constant, regulated voltage forces the current through the battery. In this method, the current at the start of the process is high but automatically tapers off, reaching a value of approximately 1 ampere when the battery is fully charged. The constant voltage method requires less time and supervision than does the constant current method.
 |
| Figure 6. Battery charging methods |
In the constant current method [Figure 6B], the current remains almost constant during the entire charging process. This method requires a longer time to charge a battery fully and, toward the end of the process, presents the danger of overcharging, if care is not exercised.
In the aircraft, the storage battery is charged by direct current from the aircraft generator system. This method of charging is the constant voltage method, since the generator voltage is held constant by use of a voltage regulator.
When a storage battery is being charged, it generates a certain amount of hydrogen and oxygen. Since this is an explosive mixture, it is important to take steps to prevent ignition of the gas mixture. Loosen the vent caps and leave in place. Do not permit open flames, sparks, or other sources of ignition in the vicinity. Before disconnecting or connecting a battery to the charge, always turn off the power by means of a remote switch.
RELATED POSTS
