Matter
Matter can be defined as anything that has mass and volume and is the substance of which physical objects are composed. Essentially, it is anything that can be touched. Matter is what all things are made of; whatever occupies space, has mass, and is perceptible to the senses in some way. Weight is an indirect method of determining mass, but it is not the same. Weight is a measure of the pull of gravity acting on the mass of an object. The more mass an object has, the more it weighs under the earth’s force of gravity. Mathematically, weight can be stated as follows:Weight = Mass × Gravity
Categories of matter are ordered by molecular activity. The four categories or states are: solids, liquids, gases, and plasma. For the purposes of the aircraft technician, only solids, liquids, and gases are considered.
Element
An element is a substance that cannot be reduced to a simpler form by chemical means. Iron, gold, silver, copper, and oxygen are examples of elements. Beyond this point of reduction, the element ceases to be what it is.Compound
A compound is a chemical combination of two or more elements. Water is one of the most common compounds and is made up of two hydrogen atoms and one oxygen atom.Molecule
The smallest particle of matter that can exist and still retain its identity, such as water (H2O), is called a molecule. [Figure 1] Substances composed of only one type of atom are called elements. But most substances occur in nature as compounds, that is, combinations of two or more types of atoms. It would no longer retain the characteristics of water if it were compounded of one atom of hydrogen and two atoms of oxygen. If a drop of water is divided and then divided again and again until it cannot be divided any longer, it is still water. |
| Figure 1. A water molecule |
Atom
The atom is considered to be the most basic building block of all matter. Atoms are composed of three subatomic particles: protons, neutrons, and electrons. These three particles determine the properties of the specific atoms. Elements are substances composed of the same atoms with specific properties. Oxygen is an example of this. The main property that defines each element is the number of neutrons, protons, and electrons. Hydrogen and helium are examples of elements. Both of these elements have neutrons, protons, and electrons but differ in the number of those items. This difference alone accounts for the variations in chemical and physical properties of these two different elements. There are over 100 known elements in the periodic table. [Figure 2] They are categorized according to their properties on that table. The kinetic theory of matter also states that the particles that make up the matter are always moving. Thermal expansion is considered in the kinetic theory and explains why matter contracts when it is cool and expands when it is hot, with the exception of water/ice.
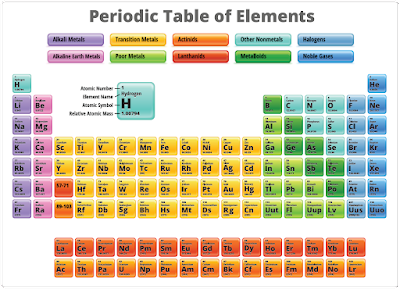 |
| Figure 2. Periodic table of elements |
Electrons, Protons, and Neutrons
At the center of the atom is the nucleus, which contains protons and neutrons. Protons are positively-charged particles, and neutrons are neutrally-charged particles. A neutron has approximately the same mass as the proton. The third particle of the atom is the electron that is a negatively-charged particle with a very small mass compared to the proton. The proton’s mass is approximately 1,837 times greater than the electron. Due to the proton and the neutron location in the central portion of the atom (nucleus) and the electron’s position at the distant periphery of the atom, it is the electron that undergoes the change during chemical reactions. Since a proton weighs approximately 1,845 times as much as an electron, the number of protons and neutrons in its nucleus determines the overall weight of an atom. The weight of an electron is not considered in determining the weight of an atom. Indeed, the nature of electricity cannot be defined clearly because it is not certain whether the electron is a negative charge with no mass (weight) or a particle of matter with a negative charge.Hydrogen represents the simplest form of an atom. [Figure 3] At the nucleus of the hydrogen atom is one proton and at the outer shell is one orbiting electron. At a more complex level is the oxygen atoms, which has eight electrons in two shells orbiting the nucleus with eight protons and eight neutrons. [Figure 4] When the total positive charge of the protons in the nucleus equals the total negative charge of the electrons in orbit around the nucleus, the atom is said to have a neutral charge.
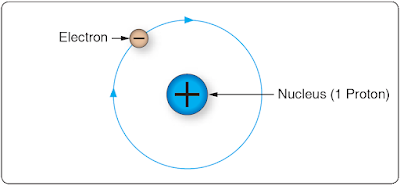 |
| Figure 3. Hydrogen atom |
 |
| Figure 4. Oxygen atom |
Electron Shells and Energy Levels
Electrons require a certain amount of energy to stay in an orbit. This particular quantity is called the electron’s energy level. By its motion alone, the electron possesses kinetic energy, while the electron’s position in orbit determines its potential energy. The total energy of an electron is the main factor that determines the radius of the electron’s orbit.Electrons of an atom appear only at certain definite energy levels (shells). The spacing between energy levels is such that when the chemical properties of the various elements are cataloged, it is convenient to group several closely spaced permissible energy levels together into electron shells. The maximum number of electrons that can be contained in any shell or sub-shell is the same for all atoms and is defined as electron capacity = 2n2. In this equation, n represents the energy level in question. The first shell can only contain two electrons; the second shell can only contain eight electrons; the third, 18, and so on until we reach the seventh shell for the heaviest atoms, which have six energy levels. Because the innermost shell is the lowest energy level, the shell begins to fill up from the shell closest to the nucleus and fill outward as the atomic number of the element increases. However, an energy level does not need to be completely filled before electrons begin to fill the next level. The Periodic Table of Elements should be checked to determine an element’s electron configuration.
[ad-mid]
Valence Electrons
Valence is the number of chemical bonds an atom can form. Valence electrons are electrons that can participate in chemical bonds with other atoms. The number of electrons in the outermost shell of the atom is the determining factor in its valence. Therefore, the electrons contained in this shell are called valence electrons.Ions
Ionization is the process by which an atom loses or gains electrons. Dislodging an electron from an atom causes the atom to become positively charged. This net positively-charged atom is called a positive ion or a cation. An atom that has gained an extra number of electrons is negatively charged and is called a negative ion or an anion. When atoms are neutral, the positively-charged proton and the negatively-charged electron are equal.Free Electrons
Valence electrons are found drifting midway between two nuclei. Some electrons are more tightly bound to the nucleus of their atom than others and are positioned in a shell or sphere closer to the nucleus, while others are more loosely bound and orbit at a greater distance from the nucleus. These outermost electrons are called “free” electrons because they can be easily dislodged from the positive attraction of the protons in the nucleus. Once freed from the atom, the electron can then travel from atom to atom, becoming the flow of electrons commonly called current in a practical electrical circuit.Electron Movement
Conductors, Insulators, and Semiconductors
The valence of an atom determines its ability to gain or lose an electron, which ultimately determines the chemical and electrical properties of the atom. These properties can be categorized as being a conductor, semiconductor, or insulator, depending on the ability of the material to produce free electrons. When a material has a large number of free electrons available, a greater current can be conducted in the material.Conductors
Elements such as gold, copper, and silver possess many free electrons and make good conductors. The atoms in these materials have a few loosely bound electrons in their outer orbits. Energy in the form of heat can cause these electrons in the outer orbit to break loose and drift throughout the material. Copper and silver have one electron in their outer orbits. At room temperature, a piece of silver wire has billions of free electrons.Insulators
Insulators are materials that do not conduct electrical current very well or not at all, such as glass, ceramic, and plastic. Under normal conditions, atoms in these materials do not produce free electrons. The absence of free electrons means that electrical current cannot be conducted through the material. Only when the material is in an extremely strong electrical field will the outer electrons be dislodged. This action is called breakdown and usually causes physical damage to the insulator.Semiconductors
The material properties of semiconductors fall in between conductors and insulators. In their pure state, they are not good at conducting or insulating. Semiconductors can operate like a conductor or insulator depending on what external load is placed on the material. Semiconductors are used to make transistors and integrated circuits. Silicon and germanium are the most widely used semiconductor materials.RELATED POSTS
