Smoke, flame, and carbon monoxide detection systems play a vital role in aircraft fire protection and occupant safety. Unlike heat-based detection systems that respond to rising temperatures, these systems are designed to sense the byproducts or radiation signatures of combustion itself. This allows for earlier warning in areas where a fire may develop slowly or where heat may not immediately reach a temperature-sensitive detector, such as lavatories, cargo compartments, cabins, and certain equipment bays.
Each type of detector operates on a different physical principle. Smoke detectors identify airborne combustion particles, flame detectors sense the specific infrared or ultraviolet radiation emitted by burning fuels, and carbon monoxide detectors monitor the presence of toxic gases produced by incomplete combustion. Together, these systems provide layered protection by detecting fire-related hazards that may not be immediately apparent through temperature monitoring alone.
Smoke Detectors
A smoke detection system monitors the lavatories and cargo baggage compartments for the presence of smoke, which is indicative of a fire condition. Smoke detection instruments that collect air for sampling are mounted in the compartments in strategic locations. A smoke detection system is used where the type of fire anticipated is expected to generate a substantial amount of smoke before temperature changes are sufficient to actuate a heat detection system. Two common types used are light refraction and ionization.
Light Refraction Type
The light refraction type of smoke detector contains a photoelectric cell that detects light refracted by smoke particles. Smoke particles refract the light to the photoelectric cell and, when it senses enough of this light, it creates an electrical current that sets off a light.
Ionization Type
Some aircraft use an ionization type smoke detector. The system generates an alarm signal (both horn and indicator) by detecting a change in ion density due to smoke in the cabin. The system is connected to the 28 volt DC electrical power supplied from the aircraft. Alarm output and sensor sensitive checks are performed simply with the test switch on the control panel.
Flame Detectors
Optical sensors, often referred to as flame detectors, are designed to alarm when they detect the presence of prominent, specific radiation emissions from hydrocarbon flames. The two types of optical sensors available are infrared (IR) and ultraviolet (UV), based on the specific emission wavelengths that they are designed to detect. IR-based optical flame detectors are used primarily on light turboprop aircraft and helicopter engines. These sensors have proven to be very dependable and economical for these applications.
When radiation emitted by the fire crosses the airspace between the fire and the detector, it impinges on the detector front face and window. The window allows a broad spectrum of radiation to pass into the detector where it strikes the sensing device filter. The filter allows only radiation in a tight waveband centered on 4.3 micrometers in the IR band to pass on to the radiation-sensitive surface of the sensing device. The radiation striking the sensing device minutely raises its temperature causing small thermoelectric voltages to be generated. These voltages are fed to an amplifier whose output is connected to various analytical electronic processing circuits. The processing electronics are tailored exactly to the time signature of all known hydrocarbon flame sources and ignores false alarm sources, such as incandescent lights and sunlight. Alarm sensitivity level is accurately controlled by a digital circuit. [Figure]
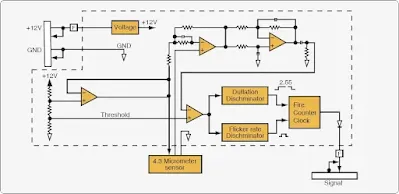 |
| Infrared (IR) based optical flame detector |
Carbon Monoxide Detectors
Carbon monoxide is a colorless, odorless gas that is a byproduct of incomplete combustion. Its presence in the breathing air of human beings can be deadly. To ensure crew and passenger safety, carbon monoxide detectors are used in aircraft cabins and cockpits. They are most often found on reciprocating engine aircraft with exhaust shroud heaters and on aircraft equipped with a combustion heater. Turbine bleed air, when used for heating the cabin, is tapped off of the engine upstream of the combustion chamber. Therefore, no threat of carbon monoxide presence is posed.
Carbon monoxide gas is found in varying degrees in all smoke and fumes of burning carbonaceous substances. Exceedingly small amounts of the gas are dangerous if inhaled. A concentration of as little as 2 parts in 10,000 may produce headache, mental dullness, and physical lethargy within a few hours. Prolonged exposure or higher concentrations may cause death.
There are several types of carbon monoxide detectors. Electronic detectors are common. Some are panel mounted and others are portable. Chemical color-change types are also common. These are mostly portable. Some are simple buttons, cards, or badges that have a chemical applied to the surface. Normally, the color of the chemical is tan. In the presence of carbon monoxide, the chemical darkens to grey or even black. The transition time required to change color is inversely related to the concentration of CO present. At 50 parts per million, the indication is apparent within 15 to 30 minutes. A concentration of 100 parts per million changes the color of the chemical in as little as 2–5 minutes. As concentration increases or duration of exposure is prolonged, the color evolves from grey to dark grey to black. If contaminated, installing a new indicating element allows a carbon monoxide portable test unit to be returned to service.
